PHYSICAL PROPERTIES OF ALKYL HALIDES a Boiling point. 2 called methylene halide eg.

Solved Draw Structures For These Alkyl And Aryl Halides Begin Array L Text A Chlorobenzene Text B 1 Text Bromo 4 Chlorohexane Text C 1 2 Text Difluoro 3 Iodocyclohexane
CH 2 Cl 2 is methylene chloride CHX 3 is a haloformeg.
. Write the name and draw the structure of the alkyl group that corresponds to 0202. The halogen atom is attached to a sp 2 hybridized carbon atom in aryl halides. Draw structures for the following molecules.
Name the following simple alkyl halides using common nomenclature. Which of these has weakest C CI bond. Only one C CH 3 X Primary Secondary and Tertiary Geminal and vicinal Nomenclature - All 16.
Classify each halo compound shown below as an alkyl vinyl or aryl halide. Cl F Br iso-butyl chloride hexyl f luoride t- buyromide 3. 3 halides 2 halides 1 halides Alkyl halides also undergo ER in the presence of base as Nu Loss of HX and formation of bond H R OR CH2 CH2 Cl ROH CH3CHCH2 Cl CH3 ii In general 3 halides tend to react by elimination.
The halogen atom is bonded to the sp 2 - hybridised carbon atom of an aromatic ring. The below chart shows the boiling point of some simple haloalkanes. Give IUPAC names for the following alkyl halides.
On the other hand aryl halides are organic compounds having halogen atoms bonded covalently to benzene rings or aromatic groups. They form a homologous series represented by C n H 2n1 X. In alkyl halides the halogen atom is bonded to an alkyl group R.
Name the type of organic compound each substance. Aryl halide Vinyl halide X F Cl Br I 101 Naming alkyl halides- Read 102 Structure of alkyl halides Table 101 Halomethane H3C-F H3C-Cl H3C-Br H3C-I Bond length pm 139 178 193 214 Bond strength KJmol 452 351 293 234 Dipole Moment 185 187 181 162 RCC _ Na CX H R H THF RCCC R H NaX The significant dipole moment of alkyl halides make then good. Define functional group and name the group present in each of the following structures.
For example benzyl chloride is not an aryl halide because halogen is not directly. The carbon-halide bond of alkyl halides has a low density of electrons compared to aryl halides. They can also be manufactured from any organic precursors such as alkanes alkenes or alcohols and carboxylic acids.
Watch More Solved Questions in Chapter 22. The order of elimination reaction is. Boiling points of haloalkanes Notice that three of these have bps below room temperature taken as.
If halogen is attached to a primary carbon atom in an alkyl halide the alkyl halide is called primary alkyl halide or 1 alkyl halide. Alkyl halides have a linear or branched structure most of the times. Alkyl and Aryl Halides of Class 12.
1 halides by substitution and 2 halides by either or both of the reactions. Draw structures for these alkyl and aryl halides Velvet manicure will make the nails manufactured like of velvet and they appear really soft for the contact. Name these alkyl groups.
Aryl halides also show dipole-dipole interactions. An aryl halide is not just any halogen compound containing an aromatic ring. The chemical reactivity of several alkyl halides is based on their structures and functions associated with them.
Draw the structures of all the alkyl halides that will be used in this experiment. The aryl halides are insoluble in water. They are further classified as primary secondary or tertiary according to the nature of carbon to which halogen is attached.
Alkyl halides are organic compounds with the general formula RX where R denotes the alkyl group and X denotes the halogen. CCl 4 is carbon tet More Classification of Alkyl Halides Methyl halides. Generally alkyl halides contain hydrogen atoms attached to the sp.
The halogen atom is bonded to an sp 2 -hybridised carbon atom of a carbon-carbon double bond. Aryl halides are always ringed structures. The same outcome is accomplished by a Specific powder flock thats sprinkled with nails.
Alkyl halide structures can be classified as primary secondary and tertiary. Name and write the structures of. Draw structures for the following molecules.
MAINDEA Compare and contrast alkyl halides and aryl halides. And also if it is allylic or benzylic. FIGURE CANNOT COPY.
Draw Structures For These Alkyl And Aryl Halides. Since the neutral bonding pattern for halogens is one bond and three lone pairs the carbon and halogen always share a single bond. Alkyl halide or haloalkanes are formed by the replacement of hydrogen atoms in an aliphatic hydrocarbon by halogen atoms Fluorine chlorine bromine or iodine.
Describe and compare the structures of alkyl halides and aryl halides. Learn vocabulary terms and more with flashcards games and other study tools. Aryl halides are compounds containing halogen attached directly to an aromatic ring.
Please draw the structures. An alkyl halide A on reaction with magnesium in dry ether followed by treatment with ethanol gave 2-. CHCl 3 is chloroform CX 4 is carbon tetrahalide.
MAINIDEA Compare and contrast alkyl halides and aryl halides. Label each as primary secondary tertiary allylic or benzylic. Consider all the primary halides in this experiment and rank them in order of reactivity in each reagent.
They are denser than water and form a separate lower layer. In these hydrocarbons one or more of the hydrogen atom s is replaced by a halogen group 17. These dipole-dipole attractions must be very unimportant relative to the dispersion forces because the most polar molecule the chlorobenzene has the lowest boiling point of the three.
The general formula of aryl halides is ArX where Ar is phenyl substituted phenyl or aryl groups. Alkyl halides are organic compounds having halogen atoms covalently attached to aliphatic carbon atoms or carbon atoms in a straight hydrocarbon orientation. BPK 400 Room 300 temperature 200 100 0 CH 3 X CH 32 CH X CH 322 CH CH X Gas Gas Chlorides Bromides Iodides Figure 121.
Alkyl halides and aryl halides are the two different types of substituted hydrocarbons compounds composed of hydrogen and carbon. If the compound is an alkyl halide indicate whether it is 1 2 or 3. Define functional group and name the group present in each of the following structures.
S N 1 S N 2 reactions. The carbon atoms here are in a cyclic orientation. In this chapter we would focus on alkyl halides and aryl halides only under following subtopics.
These alkyl halides are important because the halogens are a good leaving group that can easily react and allow the carbon to attach to something new. This is an unsaturated structure due to the presence of double bonds in the aromatic ring. Name the type of organic compound each substance.
An aryl halide is a molecule having a halogen atom attached to an sp2 hybridized carbon in an aromatic ring directly. The carbon-halogen bond is stronger than that of alkyl halides due to the presence of ring electrons.

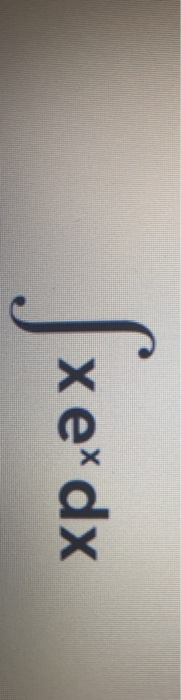
Solved Xex Dx 32 Draw Structures For These Alkyl And Aryl Chegg Com
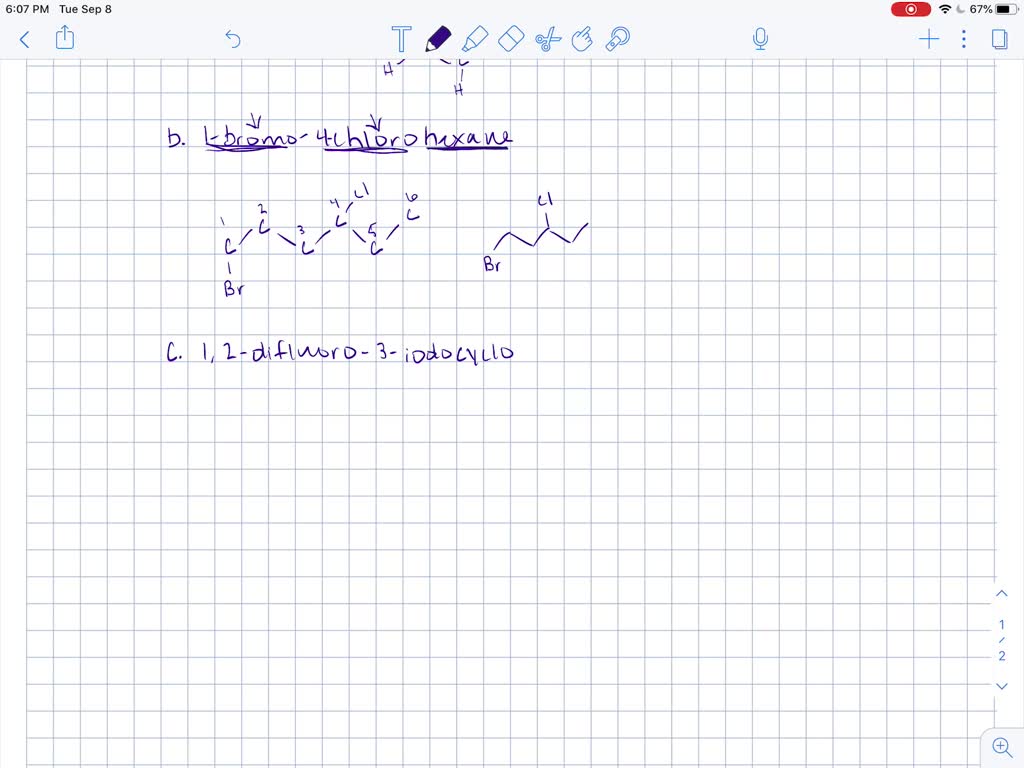
Solved Draw Structures For These Alkyl And Aryl Halides Begin Array L Text A Chlorobenzene Text B 1 Text Bromo 4 Chlorohexane Text C 1 2 Text Difluoro 3 Iodocyclohexane
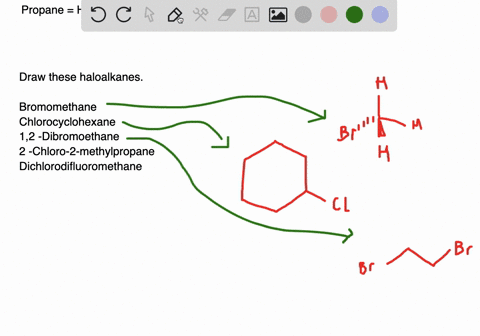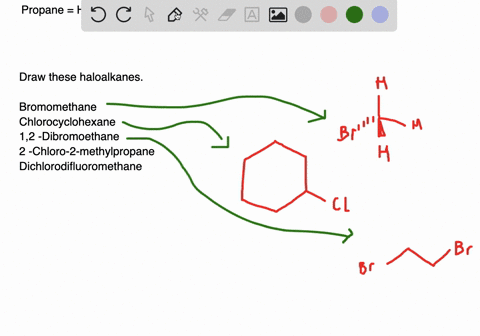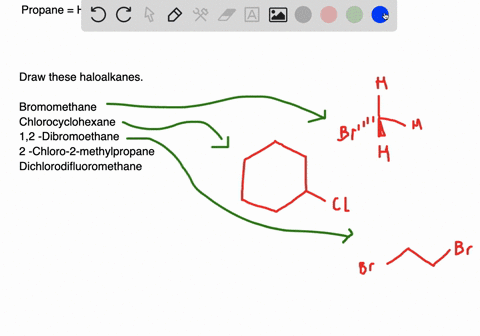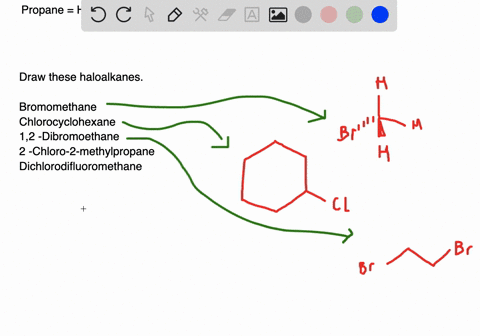
Solved Draw Structures For These Alkyl And Aryl Halides Begin Array L Text A Chlorobenzene Text B 1 Text Bromo 4 Chlorohexane Text C 1 2 Text Difluoro 3 Iodocyclohexane
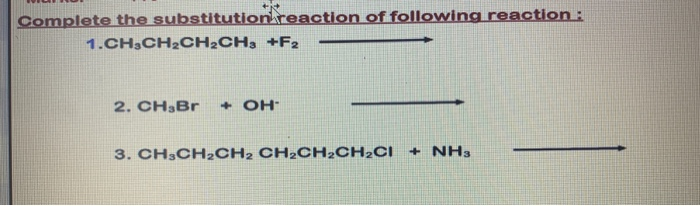
Solved 32 Draw Structures For These Alkyl And Aryl Halides Chegg Com

Solved Xex Dx 32 Draw Structures For These Alkyl And Aryl Chegg Com

Solved Draw Structures For These Alkyl And Aryl Halides Begin Equation Begin Array L Text A Chlorobenzene Text B 1 Text Bromo 4 Chlorohexane Text C 1 2 Text
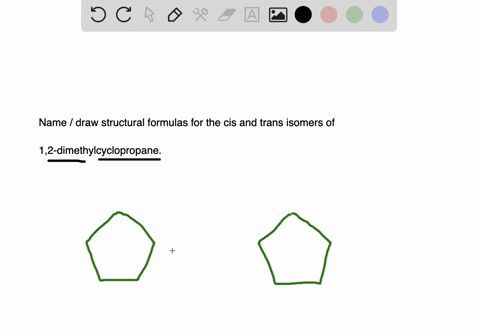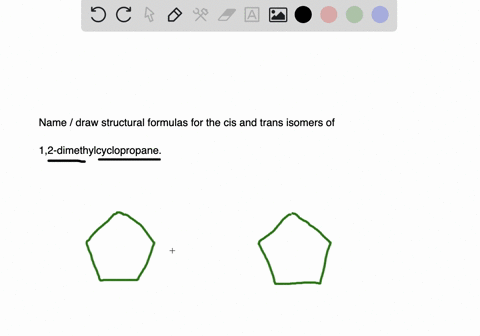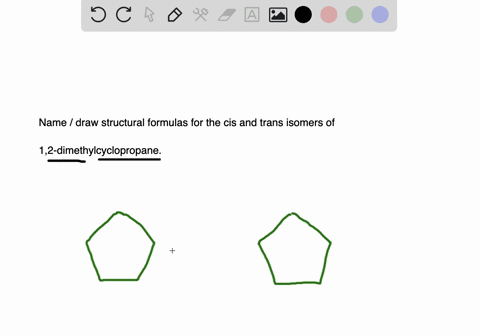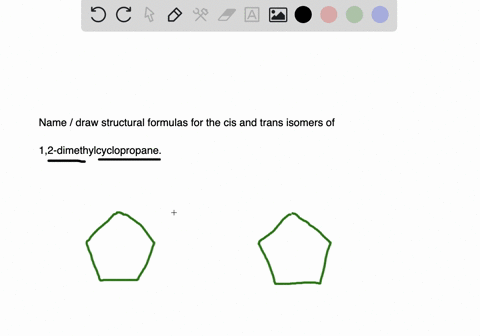
Solved Draw Structures For These Alkyl And Aryl Halides Begin Array L Text A Chlorobenzene Text B 1 Text Bromo 4 Chlorohexane Text C 1 2 Text Difluoro 3 Iodocyclohexane
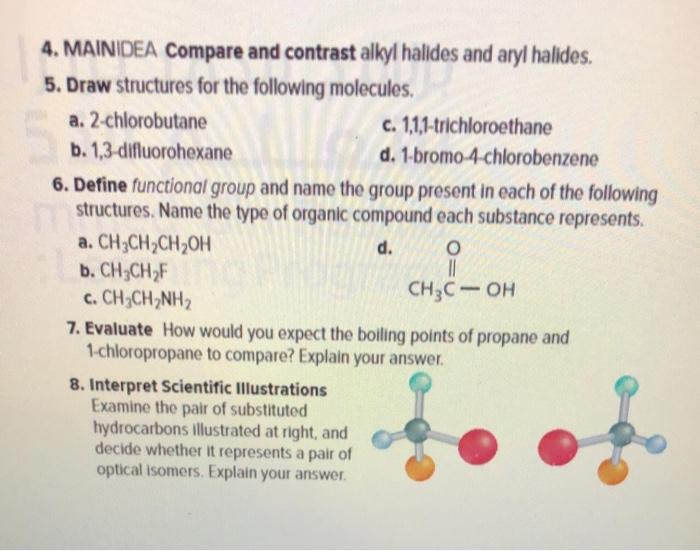
Solved 4 Maindea Compare And Contrast Alkyl Halides And Chegg Com
0 comments
Post a Comment